Answer:
B. 288. G
Step-by-step explanation:
Moles of Al :
Given, Mass of Al = 20.4 g
Molar mass of Al = 26.9815 g/mol
The formula for the calculation of moles is shown below:

Thus,
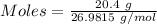
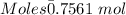
The reaction between Al and I is shown below as:
2Al + 3I₂ ⇒ 2AlI₃
2 moles of aluminium react with 3 moles of iodine
1 mole of aluminium react with 3/2 moles of iodine
0.7561 moles of aluminium react with (3/2)*0.7561 moles of iodine
Moles of Iodine = 1.13415 moles
Molar mass of NaOH = 253.8089 g/mol
The formula for the calculation of moles is shown below:

Thus,
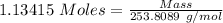
Mass of Iodine = 288 g