Answer:
Trimethylacetaldehyde
Step-by-step explanation:
For the unknow compound we have a molar mass of 86 g/mol. We have an even value for the mass, so the compound does not have nitrogen and we can have several posibilities:
A)
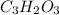
B)
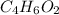
C)
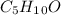
D)
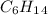
If we check the IR info a signal in 1730 cm-1 appears, this indicates that we have an oxo group (C=O). So, the D option can be discarded. The groups that can have the oxo group are: Carboxylic acids, Ketones and aldehydes.
We don have a signal in 3000 cm-1, so the carboxylic acid can be discarded. Now, is we check the info for the 1H NMR we only have 2 signals. If we only have 2 we will have a very symmetric compound.
By trial an error the find the compound Trimethylacetaldehyde (Figure 1).