Answer:
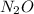 has the highest mass percent of nitrogen.
has the highest mass percent of nitrogen.
Step-by-step explanation:
To calculate the percentage composition of a nitrogen in a compound, we use the equation:
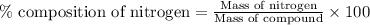 .......(1)
.......(1)
Molar mass of nitrogen = 14 g/mol
Mass of NO = 30.01 g/mol
Mass of nitrogen =
![[1* 14]=14g/mol](https://img.qammunity.org/2020/formulas/chemistry/college/xcsyh2xyvc2c0rd7ulo17socmroxc4uy3h.png)
Putting values in equation 1, we get:
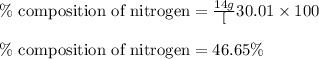
The mass percent of nitrogen in given compound is 46.65 %
- For
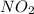 :
:
Mass of
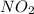 = 46.01 g/mol
= 46.01 g/mol
Mass of nitrogen =
![[1* 14]=14g/mol](https://img.qammunity.org/2020/formulas/chemistry/college/xcsyh2xyvc2c0rd7ulo17socmroxc4uy3h.png)
Putting values in equation 1, we get:
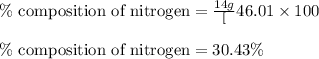
The mass percent of nitrogen in given compound is 30.43 %
- For
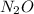 :
:
Mass of
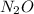 = 44.01 g/mol
= 44.01 g/mol
Mass of nitrogen =
![[2* 14]=28g/mol](https://img.qammunity.org/2020/formulas/chemistry/college/9r1oi6kz02iz1inlzcy6bkjn18l6zveq16.png)
Putting values in equation 1, we get:
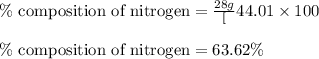
The mass percent of nitrogen in given compound is 63.62 %
- For
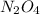 :
:
Mass of
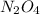 = 92.01 g/mol
= 92.01 g/mol
Mass of nitrogen =
![[2* 14]=28g/mol](https://img.qammunity.org/2020/formulas/chemistry/college/9r1oi6kz02iz1inlzcy6bkjn18l6zveq16.png)
Putting values in equation 1, we get:
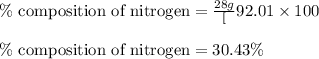
The mass percent of nitrogen in given compound is 30.43 %
Hence,
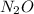 has the highest mass percent of nitrogen.
has the highest mass percent of nitrogen.