Answer:
The formula weight of the salt is 187.46 g/mol.
Step-by-step explanation:
Let the formula weight of the salt = M
Number of copper atom per formula unit = 1
Atomic mass of copper = 63.55 g/mol
Percentage of copper in single formula unit = 33.9%
Percentage of an element in a compound:
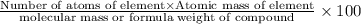
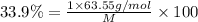
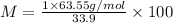
M =187.46 g/mol
The formula weight of the salt is 187.46 g/mol.