Step-by-step explanation:
dU=TdS-pdV (given)
To prove = 1)
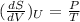 (at constant U)
(at constant U)
2)
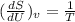 (at constant V)
(at constant V)
Solution: 1)
dU=TdS-PdV
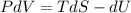
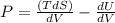
Derivative of constant is zero.
Given that internal energy is ,U = constant
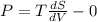
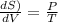 (hence proved)
(hence proved)
Solution: 2)
dU=TdS-PdV
Differentiating with respect to dU, we get:
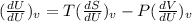
Derivative of constant is zero.
Given that volume is constant , V= constant
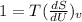
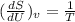 (hence proved)
(hence proved)