Step-by-step explanation:
Atomic radius of an atom is defined as the total distance from the nucleus to the outermost shell of the atom.
An ion is formed when a neutral atom looses or gains electrons.
When an atom looses electrons, it results in the formation of positive ion known as cation.
When an atom gains electrons, it results in the formation of negative ion known as anion.
As moving from left to right in a period, more and more electrons get added up in the same shell and the attraction between the last electron and nucleus increases, which results in the shrinkage of size of an atom. Hence, the size of an atom decreases.
The size of the cation is small then their neutral atom because it has less number of electrons while its nuclear charge remains the same. Thus, the nucleus attracts the electron more towards itself and leads to the decrease in size.
We are given three cations:
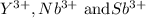
Yttrium lies in Period 5, group 3 of the periodic table.
Electronic configuration of Yttrium
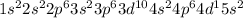
Niobium lie in Period 5, group 5 of the periodic table.
Electronic configuration of Niobium
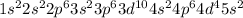
Antimony lies in Period 5, group 15 of the periodic table.
Electronic configuration of Antimony
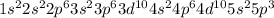
So, the order of atomic radii in increasing order follows:
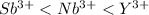
Hence, antimony ion is the smallest and yttrium ion is the largest.