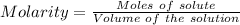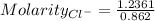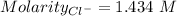Answer:
Step-by-step explanation:
Moles of NaCl :
Given, Mass of NaCl = 72.24 g
Molar mass of NaCl = 58.44 g/mol
The formula for the calculation of moles is shown below:
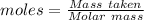
Thus,
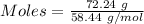
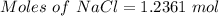
NaCl is a strong salt. So, moles of chloride ion = 1.2361 moles
Volume = 0.862 L