Answer:
12.29 M
Step-by-step explanation:
- The reaction that takes place is:
H₂SO₄ + 2NaOH → 2Na⁺ + SO₄⁻² + 2H₂O
- Now let's calculate the moles of H₂SO₄ that were titrated:
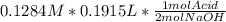 = 0.01229 mol H₂SO₄.
= 0.01229 mol H₂SO₄.
- Thus, the concentration of the diluted solution is:
0.01229 mol H₂SO₄ / 0.010 L = 1.229 M
- Finally, the concentration of the original acid solution is:
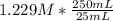 = 12.29 M
= 12.29 M