Answer: The increase in pressure is 0.003 atm
Step-by-step explanation:
To calculate the final pressure, we use the Clausius-Clayperon equation, which is:
![\ln((P_2)/(P_1))=(\Delta H)/(R)[(1)/(T_1)-(1)/(T_2)]](https://img.qammunity.org/2020/formulas/chemistry/college/usutkvlx1bir5fna1wj6ube6r9t7dsccsn.png)
where,
 = initial pressure which is the pressure at normal boiling point = 1 atm
= initial pressure which is the pressure at normal boiling point = 1 atm
 = final pressure = ?
= final pressure = ?
 = Enthalpy change of the reaction = 28.8 kJ/mol = 28800 J/mol (Conversion factor: 1 kJ = 1000 J)
= Enthalpy change of the reaction = 28.8 kJ/mol = 28800 J/mol (Conversion factor: 1 kJ = 1000 J)
R = Gas constant = 8.314 J/mol K
 = initial temperature =
= initial temperature =
![801^oC=[801+273]K=1074K](https://img.qammunity.org/2020/formulas/chemistry/college/vgtyw9km9la6wh9e8doeg2l7cub6l6blx7.png)
 = final temperature =
= final temperature =
![(801+1.00)^oC=802.00=[802+273]K=1075K](https://img.qammunity.org/2020/formulas/chemistry/college/lsmtswyjbw8c5du9nilpu392p47i6ykurl.png)
Putting values in above equation, we get:
![\ln((P_2)/(1))=(28800J/mol)/(8.314J/mol.K)[(1)/(1074)-(1)/(1075)]\\\\\ln P_2=3* 10^(-3)atm\\\\P_2=e^{3* 10^(-3)}=1.003atm](https://img.qammunity.org/2020/formulas/chemistry/college/ija1he0icnr5gml9or9a68wciohjryfxmg.png)
Change in pressure =
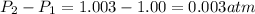
Hence, the increase in pressure is 0.003 atm