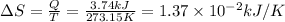Answer:
ΔS = 1.37 × 10⁻² kJ/K
Step-by-step explanation:
The change in entropy (ΔS) for the melting process can be calculated using the following expression.
ΔS = Q/T [1]
where,
Q is the heat absorbed by the block of ice
T is the absolute temperature (in this case 0°C = 273.15K)
Q can be calculated like:
Q = ΔHfus . n = ΔHfus . m / M
where,
ΔHfus is the enthalpy of fusion for water
n is the number of moles
m is the mass
M is the molar mass
Then,
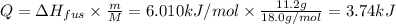
We can replace this value in [1].