Answer :
(1)
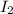 act as a reducing agent and
act as a reducing agent and
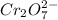 act as a oxidizing agent.
act as a oxidizing agent.
(2)
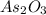 act as a reducing agent and
act as a reducing agent and
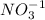 act as a oxidizing agent.
act as a oxidizing agent.
Explanation :
Reducing agent : It is defined as the agent which helps the other substance to reduce and itself gets oxidized. Thus, it will undergo oxidation reaction.
Oxidizing agent : It is defined as the agent which helps the other substance to oxidize and itself gets reduced. Thus, it will undergo reduction reaction.
(1)
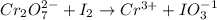
In this reaction, 'I' is oxidized from oxidation (0) to (+5) and 'Cr' is reduced from oxidation state (+6) to (+3). Hence,
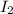 act as a reducing agent and
act as a reducing agent and
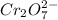 act as a oxidizing agent.
act as a oxidizing agent.
(2)
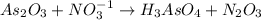
In this reaction, 'As' is oxidized from oxidation (+3) to (+5) and 'N' is reduced from oxidation state (+5) to (+3). Hence,
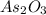 act as a reducing agent and
act as a reducing agent and
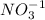 act as a oxidizing agent.
act as a oxidizing agent.