Answer : The pH of the solution is, 4.9
Explanation : Given,
Dissociation constant for acetic acid =
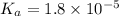
Concentration of acetic acid = 0.05 M
Concentration of sodium acetate = 0.075 M
First we have to calculate the value of
 .
.
The expression used for the calculation of
 is,
is,
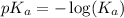
Now put the value of
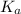 in this expression, we get:
in this expression, we get:
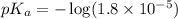
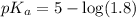
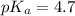
Now we have to calculate the pH of buffer.
Using Henderson Hesselbach equation :
![pH=pK_a+\log ([Salt])/([Acid])](https://img.qammunity.org/2020/formulas/chemistry/college/6wyuhr9b7n0qwlgrnwgg688yylfbvv3wby.png)
![pH=pK_a+\log ([CH_3COONa])/([CH_3COOH])](https://img.qammunity.org/2020/formulas/chemistry/college/koy73tgof7x26myuk57xza5rphisi7b7zj.png)
Now put all the given values in this expression, we get:
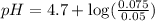
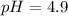
Therefore, the pH of the solution is 4.9.