Answer:
-586.56 kJ/mol is the standard enthalpy of the 3rd reaction.
Step-by-step explanation:
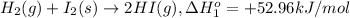 ...[1]
...[1]
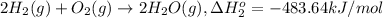 ...[2]
...[2]
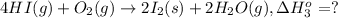 ..[3]
..[3]
The unknown standard enthalpy of third reaction can be calculated by using Hess's law:
The law states that 'the heat absorbed or evolved in a given chemical equation is the same whether the process occurs in one step or several steps'.
[2] - 2 × [1] = [3]
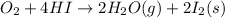
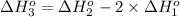
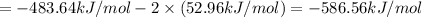
The standard enthalpy of the 3rd reaction is -586.56 kJ/mol.The negative sign indicates that energy is released during this reaction.