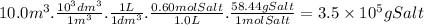Answer:
3.5 × 10⁵ g of salt
Step-by-step explanation:
What is the mass (grams) of salt in 10.0 m³ of ocean water?
We have this data:
- 1.000 mol salt is equal to 58.44 g salt
- 1.0 L of ocean water contains 0.60 mol of salt
We will need the following relations:
We can use proportions: