Answer: The percent composition of
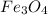 in taconite is 37.6 %.
in taconite is 37.6 %.
Step-by-step explanation:
We are given:
Mass of taconite pellets = 1 ton = 907185 g (Conversion factor: 1 ton = 907185 g)
Mass of iron produced = 545 lb = 247212 g (Conversion factor: 1 lb = 453.6 g )
We know that:
Molar mass of iron = 55.85 g/mol
Molar mass of
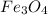 = 231.53 g/mol
= 231.53 g/mol
1 mole of
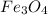 contains 3 moles of iron atom and 4 moles of oxygen atom
contains 3 moles of iron atom and 4 moles of oxygen atom
(3 × 55.85) = 167.55 g of iron is produced from 231.53 grams of
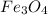
So, 247212 grams of iron will be produced from =
 of
of
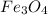
To calculate the percentage of
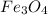 in taconite, we use the equation:
in taconite, we use the equation:
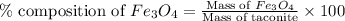
Mass of taconite = 907185 g
Mass of
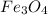 = 341611.43 g
= 341611.43 g
Putting values in above equation, we get:
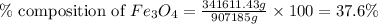
Hence, the percent composition of
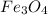 in taconite is 37.6 %.
in taconite is 37.6 %.