Answer:
The standard enthalpy of the 3rd reaction is -586.56 kJ/mol.
Step-by-step explanation:
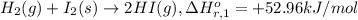 ...[1]
...[1]
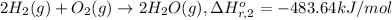 ...[2]
...[2]
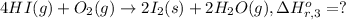 ..[3]
..[3]
By using Hess's we can determine the standard enthalpy of reaction of third reaction:
[2] - 2 × [1] = [3] (Hess's law)
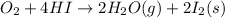

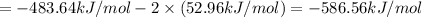
The negative sign indicates that energy is released during this reaction.
The standard enthalpy of the 3rd reaction is -586.56 kJ/mol.