Step-by-step explanation:
Formula to calculate the Debye-Hückel screening length is as follows.
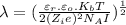 ......... (1)
......... (1)
where,
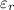 = dielectric constt. of water = 78.5
= dielectric constt. of water = 78.5
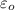 = permitivity of space =
= permitivity of space =
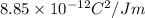
T = temperature = 298 K
e = charge on electron =
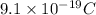
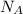 = Avogadro's number =
= Avogadro's number =
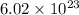 ions/mol
ions/mol
I = ionic activity = 0.001 molal
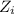 = charge on the species = 1 (in case of both
= charge on the species = 1 (in case of both
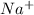 and
and
 )
)
Also, I =
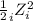
=
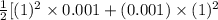
= 0.001 molal
Now, putting all the given values into equation (1) as follows.
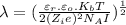
=
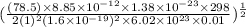
=
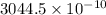 m
m
=
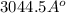
Thus, we can conclude that Debye-Hückel screening length in given solution is
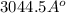 .
.