Answer:
It would be better to use the NaCl solution.
Step-by-step explanation:
We can calculate the amount of Cl⁻ in each salt.
NaCl
Taking into account the molar masses, there are 35.5 g of Cl⁻ every 58.5g of NaCl. So, for a 23 w/w% solution of NaCl:
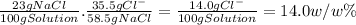
CaCl₂
Taking into account the molar masses, there are 71.0 g of Cl⁻ every 111g of CaCl₂. So, for a 23 w/w% solution of CaCl₂:
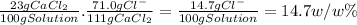
The concentration of Cl⁻ in the NaCl solution is 14.0 w/w% and its concentration in the CaCl₂ solution is 14.7 w/w%, so we would choose the NaCl solution to minimize Cl⁻ concentration.