Answer: Option (d) is the correct answer.
Step-by-step explanation:
It is known that NaCl is an ionic compound as it is formed by transfer of an electron from sodium atom to chlorine atom.
Since, an ionic bond is a bond formed due to transfer or donation of an electron from one atom to another.
Also, ionic compounds are soluble in polar solvents because like dissolves like.
So, when NaCl is added into water then it will dissociate into ions as
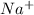 and
and
 . As a result, due to the opposite charges there will occur strong ion dipole attractions between these ions and water molecules.
. As a result, due to the opposite charges there will occur strong ion dipole attractions between these ions and water molecules.
Whereas kerosene comes from petroleum (non-polar hydrocarbon) therefore, kerosene is a non-polar solvent. Hence, NaCl will not dissolve in it.
Thus, we can conclude that NaCl will be more soluble in water, because water is polar and can separate the cation and anion in the lonic compound, creating very strong ion dipole attractions.