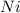Answer:
Average atomic mass = 58.51047 amu
The symbol is
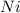
Step-by-step explanation:
The formula for the calculation of the average atomic mass is:

Given that:
For first isotope:
% = 68.274 %
Mass = 57.9353 amu
For second isotope:
% = 26.095 %
Mass = 58.9332 amu
For third isotope:
% = 1.134 %
Mass = 61.9283 amu
For fourth isotope:
% = 3.593 %
Mass = 63.9280 amu
For fifth isotope:
% = 0.904 %
Mass = 63.9280 amu
Thus,
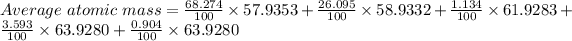
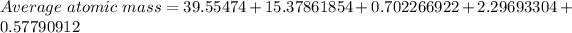
Average atomic mass = 58.51047 amu
The symbol is