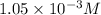Answer : The molarity of the ion is
Explanation : Given,
Density of sample = 1.00 g/mL
Concentration of
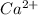 = 42 mg/kg
= 42 mg/kg
First we have to calculate the volume of sample.
Let us assume that the mass of sample be 1 kg or 1000 g.

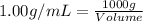
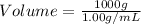
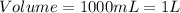 (1 L = 1000 mL)
(1 L = 1000 mL)
Now we have to calculate the moles of
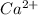
As we are given that,
Mass of
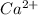 in 1 kg of sample = 42 mg = 0.042 g
in 1 kg of sample = 42 mg = 0.042 g
Molar mass of Ca = 40 g/mole
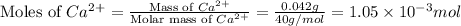
Now we have to calculate the molarity of the ion.
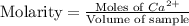
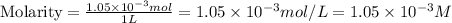
Therefore, the molarity of the ion is