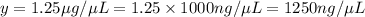Answer:
The final concentration in terms μg / μL is
 .
.
The final concentration in terms μg / nL is
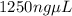 .
.
Step-by-step explanation:
Initial concentration = 25 μg / μL
diluted concentration of 25 μg / μL by 1:2 = x
Ratio of final concentration to initial concentration = 1:2
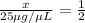
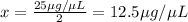
Final concentration after dilution of x by 1:10 = y
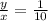
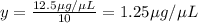
1 μg = 1000 ng