Answer :
(a) The concentration of
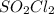 after 1.00 hr will be 0.0037 M
after 1.00 hr will be 0.0037 M
(b) The concentration of
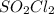 after 1.00 day will be 0.00060 M
after 1.00 day will be 0.00060 M
Explanation :
(a) The expression for first order reaction is:
![[C_t]=[C_o]e^(-kt)](https://img.qammunity.org/2020/formulas/chemistry/college/rft9amygnrxaj7fh268osrn1mx1svyzwy0.png)
where,
![[C_t]](https://img.qammunity.org/2020/formulas/chemistry/college/16n44kmdhlor0i462nohp7yv7f7u48xm7l.png) = concentration of
= concentration of
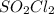 at time 't' = ?
at time 't' = ?
![[C_o]](https://img.qammunity.org/2020/formulas/chemistry/college/4nzv6ljvpvgub5bmzbof8ig137jttud8kf.png) = concentration of
= concentration of
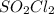 at time '0' = 0.0040 M
at time '0' = 0.0040 M
k = rate constant =
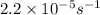 (assuming the power of 10 from the correct source)
(assuming the power of 10 from the correct source)
t = time = 1.00 hr = 3600 s (1 hr = 3600 s)
Now put all the given values in the above expression, we get:
![[C_t]=(0.0040)* e^{-(2.2* 10^(-5))* (3600)}](https://img.qammunity.org/2020/formulas/chemistry/college/yw79yin8dghtztr3x08vw3icj1q1es0rly.png)
![[C_t]=0.0037M](https://img.qammunity.org/2020/formulas/chemistry/college/dlsnwr0joh5n9m2fzztmsru7iuddwr1ee0.png)
Thus, the concentration of
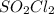 after 1.00 hr will be 0.0037 M
after 1.00 hr will be 0.0037 M
(b) The expression for first order reaction is:
![[C_t]=[C_o]e^(-kt)](https://img.qammunity.org/2020/formulas/chemistry/college/rft9amygnrxaj7fh268osrn1mx1svyzwy0.png)
where,
![[C_t]](https://img.qammunity.org/2020/formulas/chemistry/college/16n44kmdhlor0i462nohp7yv7f7u48xm7l.png) = concentration of
= concentration of
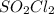 at time 't' = ?
at time 't' = ?
![[C_o]](https://img.qammunity.org/2020/formulas/chemistry/college/4nzv6ljvpvgub5bmzbof8ig137jttud8kf.png) = concentration of
= concentration of
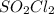 at time '0' = 0.0040 M
at time '0' = 0.0040 M
k = rate constant =
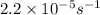
t = time = 1.00 day = 86400 s (1 day = 86400 s)
Now put all the given values in the above expression, we get:
![[C_t]=(0.0040)* e^{-(2.2* 10^(-5))* (86400)}](https://img.qammunity.org/2020/formulas/chemistry/college/k4fms1iagz133anfo9amc09z185dgjm1wx.png)
![[C_t]=0.00060M](https://img.qammunity.org/2020/formulas/chemistry/college/jsf51zq9y6qsxf74cd7u2q0z8tli8u68d4.png)
Thus, the concentration of
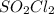 after 1.00 day will be 0.00060 M
after 1.00 day will be 0.00060 M