Answer: The soil will be
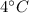 warmer than the water.
warmer than the water.
Step-by-step explanation:
The heat (thermal energy) absorbed can be found using the following equation:
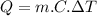
Where:
 is the heat
is the heat
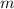 is the mass of the element
is the mass of the element
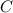 is the specific heat capacity of the material.
is the specific heat capacity of the material.
 is the variation in temperature
is the variation in temperature
In the case of soil we have:
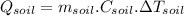 (1)
(1)
Where:
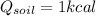
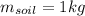
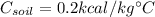
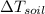
In the case of water we have:
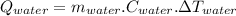 (2)
(2)
Where:
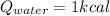
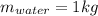
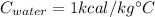
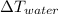
Isolating
 from both equations:
from both equations:
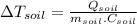 (3)
(3)
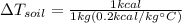
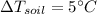 (4)
(4)
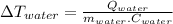 (5)
(5)
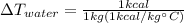
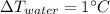 (6)
(6)
Comparing (4) and (6) we can find the soil will be
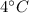 warmer than the water.
warmer than the water.