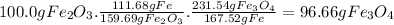Answer:
96.66 g of Fe₃O₄
Step-by-step explanation:
In order to calculate the weight of Fe₃O₄ in 100.0 g of Fe₂O₃ we need elements in common between both substances, for instance, the mass of Fe in each one. We will use the molar mass of Fe = 55.84g/mol The conversion factors we need are:
- 159.69 g of Fe₂O₃ contain 2 × 55.84 = 111.68 g of Fe
- 231.54 g of Fe₃O₄ contain 3 × 55.84 = 167.52 g of Fe
Then, we can use proportions: