Step-by-step explanation:
It is given that,
Length of iron bar, l = 14 m
Room temperature, T = 20° C
Pressure, P = 1 atm
The change in length due to change in temperature is called linear expansion. It is given by :
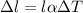
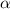 is the coefficient of linear expansion,
is the coefficient of linear expansion,
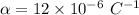
We know that the boiling temperature of water T' = 100° C
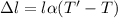
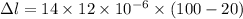
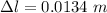
So, the new length of the iron rod is,
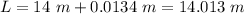
Hence, this is the required solution.