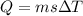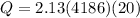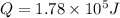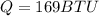Answer:
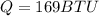
Step-by-step explanation:
As we know that volume is given as
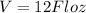
so it is given in liter as
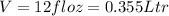
now we have six pack of such volume
so total volume is given as
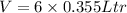
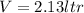
so its mass is given as
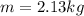
now the change in temperature is given as
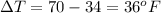
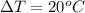
now the heat given to the liquid is given as