Answer:
The mass of the water vapor in the apartment would be 22.1 kg
Step-by-step explanation:
The ideal gas equation would be used to obtain the total mass and is expressed as;
P V = n R T .............1
but n = m/M
Substituting into equation 1 we have;
P V = (m/M)RT
Making m the subject formula we have;
m = M P V / R T ..............................3
Where M is the molar mass of water = 18 g/ mol
NB the pressure of water at
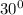 C from the saturation table is 4250 Pa
C from the saturation table is 4250 Pa
P is the pressure at 52% relative humidity = 0.52 x 4250 =2210 Pa
V is the volume = 20 x 10 x 7 = 1400
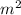
T is the temperature, T =
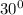 C + 273.15 = 303.15 K
C + 273.15 = 303.15 K
R is the ideal gas constant = 8.3245 J/mol/K
m = (18 g/ mol x 2210 Pa x 1400
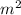 )/ (8314 J x 303.15 K)
)/ (8314 J x 303.15 K)
m = 55692000 / 2520.3891
m =22096.58 g
m = 22096.58/1000
m = 22.1 kg
Therefore the mass of the water vapor in the apartment would be 22.1 kg