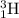Answer: The isotopic representation of tritium atom is
Step-by-step explanation:
Isotopes are defined as the chemical species which have same atomic number but differ in mass number.
Hydrogen has 3 isotopes that occur naturally. They are: H-1 (protium), H-2 (deuterium) and H-3 (tritium)
The isotopic representation of an atom is:
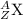
where,
Z = Atomic number of the atom
A = Mass number of the atom
X = Symbol of the atom
We know that:
Mass number of H-3 isotope = 3
Atomic number of H-3 isotope = 1
Hence, the isotopic representation of tritium atom is