Answer:
The thermal energy released per gram is 19.2 kJ/g.
(3) is correct option.
Step-by-step explanation:
Given that,
Weight of hydrocarbon = 2.50 g
Heat capacity
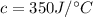
We need to calculate the thermal energy released
Using formula of thermal energy
Heat released =heat absorb by calorimeter+heat absorb by water
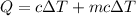
Put the value into the formula
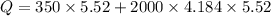
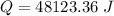
Now, The thermal energy released per gram
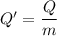
Put the value into the formula
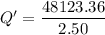
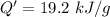
Hence, The thermal energy released per gram is 19.2 kJ/g.