Answer:
48 µg of the complex should be added when a drug company is making a 6 kg batch of vitamins.
Explanation:
Given : The concentration of Vitamin B complex in a certain vitamin mixture is 8 ppb.
To find : How many µg of the complex should be added when a drug company is making a 6 kg batch of vitamins?
Solution :
The formula used to find the mass is given by,
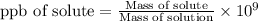
ppb denotes parts per billion.
We have given, concentration of vitamin B complex is 8 ppb.
Mass of solution is 6 kg =
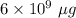
(As
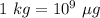 )
)
Substitute in the formula,
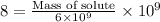
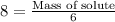
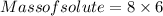
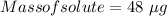
Therefore, 48 µg of the complex should be added when a drug company is making a 6 kg batch of vitamins.