Answer:
It should be used 0.08 g of drug A to prepare 100 mL of a 1:1250 solution.
Explanation:
1:1250 solution can also be expressed as: 1 g A/ 1250 ml solution=0.08% W/V
Hence:
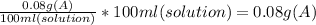
In conclusion, we have to weigh 0.08 g of drug A and dilute until getting 100 ml of solution.