Answer:
We need 9.045 grams of active ingredient F to prepare a 6.7% (w/w) solution in water.
Explanation:
6.7% (w/w) solution in water can also be expressed as: 6.7 g in 100 g of solution.
Hence:
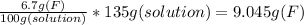
We have to dilute 9.045 g of active ingredient F until getting 100 g of solution.