Synthesis Reactions: Formation of water from hydrogen and oxygen, Formation of sodium chloride from sodium and chlorine, Formation of potassium chloride from potassium and chlorine, Formation of iron oxide from iron and oxygen. Decomposition Reaction: Decomposition of water into hydrogen and oxygen, Decomposition of calcium carbonate into calcium oxide and carbon dioxide.
Here are the chemical equations categorized as synthesis and decomposition reactions:
Synthesis Reactions:
1.
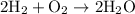 (Formation of water from hydrogen and oxygen)
(Formation of water from hydrogen and oxygen)
2.
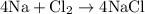 (Formation of sodium chloride from sodium and chlorine)
(Formation of sodium chloride from sodium and chlorine)
3.
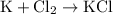 (Formation of potassium chloride from potassium and chlorine)
(Formation of potassium chloride from potassium and chlorine)
4.
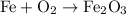 (Formation of iron oxide from iron and oxygen)
(Formation of iron oxide from iron and oxygen)
Decomposition Reaction:
1.
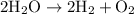 (Decomposition of water into hydrogen and oxygen)
(Decomposition of water into hydrogen and oxygen)
2.
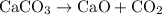 (Decomposition of calcium carbonate into calcium oxide and carbon dioxide)
(Decomposition of calcium carbonate into calcium oxide and carbon dioxide)