Answer: The correct answer is Option d.
Step-by-step explanation:
Electronegativity is defined as the tendency of an element to attract the shared pair of electron towards itself in a compound.
When electron is transferred from less electronegative atom to more electronegative atom.
The atom which is more electronegative gains electron and undergoes reduction reaction.
Reduction reaction is defined as the reaction in which an atom gains electrons. The oxidation number of the atom gets reduced during this reaction.
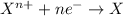
During the transfer of electrons, energy is released when an electron looses is potential energy during the transfer.
Hence, the correct answer is Option d.