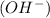Answer:
13.

This is a double displacement reaction, not an acid-base reaction
14.
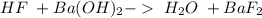
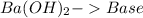
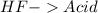
15.

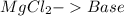
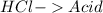
16.
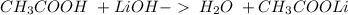
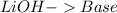
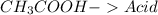
17.
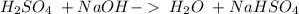

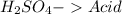
18.
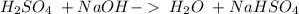

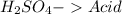
19.
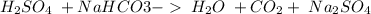

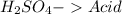
20.
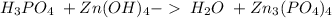
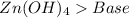
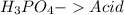
21.
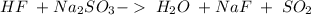
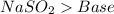
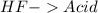
22.
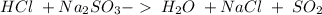
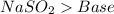
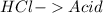
23.
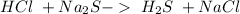
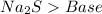
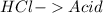
24.
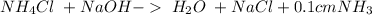
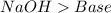
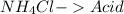
Step-by-step explanation:
All acid-base reaction have the same products,
 and a salt. Whit this in mind the acids would the compounds that produces the hydronium ion
and a salt. Whit this in mind the acids would the compounds that produces the hydronium ion
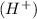 and the bases would be the compounds that produces the hydroxide ion
and the bases would be the compounds that produces the hydroxide ion