Answer:
For A: The chemical equation is
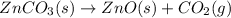
For B: The enthalpy of the reaction is 71.5 kJ/mol
Step-by-step explanation:
Decomposition reaction is defined as the reaction in which a single chemical substance breaks down into two or more smaller substances.
The chemical equation for the decomposition of zinc carbonate follows:
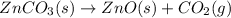
Endothermic reactions are defined as the reactions in which energy is absorbed in the reaction. The enthalpy change of the reaction is positive.
Exothermic reactions are defined as the reactions in which energy is released in the reaction. The enthalpy change of the reaction is negative.
In the above reaction, the heat is added. So, the reaction is endothermic in nature.
Amount of heat added per mole of zinc carbonate = 71.5 kJ
Hence, the enthalpy of the reaction is 71.5 kJ/mol