Answer:
The value of an integer x in the hydrate is 10.
Step-by-step explanation:
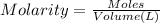
Molarity of the solution = 0.0366 M
Volume of the solution = 5.00 L
Moles of hydrated sodium carbonate = n
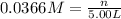
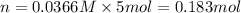
Mass of hydrated sodium carbonate = n= 52.2 g
Molar mass of hydrated sodium carbonate = 106 g/mol+x18 g/mol
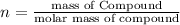
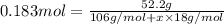
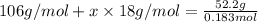
Solving for x, we get:
x = 9.95 ≈ 10
The value of an integer x in the hydrate is 10.