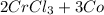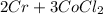 →
→
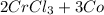
Step-by-step explanation:
- The products formed are chromic chloride and cobalt.
Chromium + Cobaltous Chloride = Chromic Chloride + Cobalt
- Type of reaction is Single Displacement (Substitution) which is there is a displacement of one atom.
Reactants used in the reaction are -
- Chromium
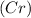
- Cobaltous Chloride
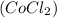
Products formed in the reaction are -
- Chromic Chloride
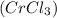
- Cobalt
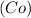
Hence, the chemical reaction is as follows -
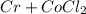 →
→
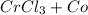
For balancing the above chemical equation we need to add a coefficient of 2 in front of chromium and of 3 in front of cobalt(II)chloride on right-hand-side while of 2 in front of chromium chloride and of 3 in front of carbon monoxide on left-hand-side of the equation.
Hence, the balanced equation is -
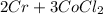 →
→