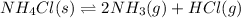The question is incomplete, here is the complete question:
Which formula equation shows a reversible reaction?
A:
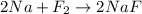
B:

C:
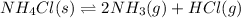
D:
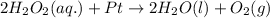
Answer: The reversible reaction is
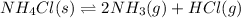
Step-by-step explanation:
Reversible reaction is defined as the reaction in which the products formed react together to give the reactants back. For general reversible chemical equation:
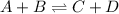
Irreversible reaction is defined as the reaction in which the reactants only lead to products and the reaction do not proceed back. For general irreversible chemical reaction:
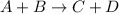
For the given options:
The reaction having an equilibrium sign between the reactants and products is equation C.
Hence, the reversible reaction is