When 25.6 g
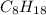 is burned, the moles of
is burned, the moles of
 emitted into the atmosphere is 1.80 mol
emitted into the atmosphere is 1.80 mol
Step-by-step explanation:
The molar mass of octane
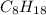 is calculated as 8(12) +18(1) = 114 g/mol.
is calculated as 8(12) +18(1) = 114 g/mol.
Where atomic mass of Carbon is C=12, and Hydrogen H=1.The sample contains 25.6 g of octane, Therefore
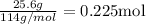
The combustion of octane can be written as:
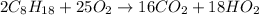
The mole ratio can be represented as:
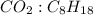 = 16:2 = 8:1.
= 16:2 = 8:1.
Hence, the quantity of carbon dioxide emitted is:
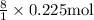 = 1.80 mol
= 1.80 mol