Answer:
Approximately 340.2 joules.
Step-by-step explanation:
The specific heat capacity of a substance gives the amount of heat required to raise the temperature of unit mass of that substance by unit temperature. Look up the specific heat capacity of iron in a thermodynamic data sheet.
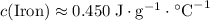 , where
, where
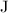 (joule) is the unit for heat/energy,
(joule) is the unit for heat/energy,
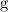 (gram) is the unit for mass, and
(gram) is the unit for mass, and
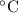 degree celsius is the unit for temperature.
degree celsius is the unit for temperature.
In other words, it takes approximately
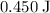 of energy to raise the temperature of one gram of iron by one degree celsius.
of energy to raise the temperature of one gram of iron by one degree celsius.
In this question,
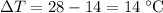 .
.
Apply the equation:
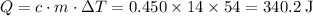 .
.