Answer:
The cube A is magnesium, the cube B is aluminum and the cube C is silver.
Step-by-step explanation:
Density is defined by the expression
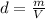 where m is the mass and V is the volume, therefore:
where m is the mass and V is the volume, therefore:
- Density of the cube A:
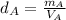
- Density of the cube B:
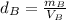
- Density of the cube C:
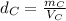
Solving for mass:
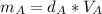
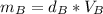
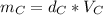
And all the three cubes have the same mass, so:
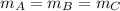
Therefore:
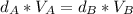 (Eq.1)
(Eq.1)
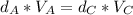 (Eq.2)
(Eq.2)
Solving for
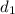 in Eq.1:
in Eq.1:
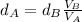
Replacing values for the volume:
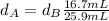
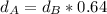
As we know the density of the aluminum is
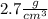 , so replacing this value for
, so replacing this value for
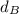 :
:
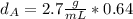
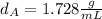
that is the density of the magnesium.
Solving for
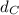 in Eq.2:
in Eq.2:
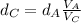
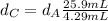
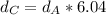
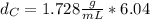
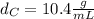
That is the density of the silver.
Therefore the cube A is magnesium, the cube B is aluminum and the cube C is silver.