Step-by-step explanation:
The given data is as follows.
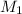 = 3 M,
= 3 M,
 = ?
= ?
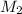 = 10 M,
= 10 M,
 = 10.0 ml
= 10.0 ml
Therefore, calculate the volume of given solution as follows.
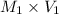 =
=
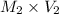
Now, putting the given values into the above formula as follows.
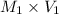 =
=
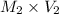
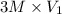 =
=
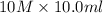
 = 33.3 ml
= 33.3 ml
or, = 0.033 L (as 1 ml = 0.001 L)
Since, molarity of NaOH is given as 3 M and its volume is calculated as 33.3 ml.
Molarity =
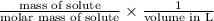
3 M =
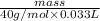
mass = 3.96 g
Therefore, we can conclude that mass of NaOH is 3.96 g.