Answer:
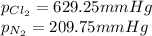
Step-by-step explanation:
Hello,
At first, consider the chemical reaction:
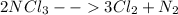
Now, considering the ideal gas equation, we compute the total moles:
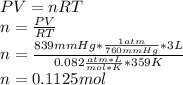
Then, taking into account that the total moles are stoichiometrically handed out by the half (0.05625mol), one can say that:
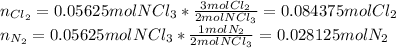
Thus, the molar fractions are:

Finally, the partial pressures are:
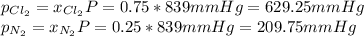
Best regards.