Step-by-step explanation:
Speed (c) will be calculated as follows.
c =
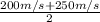 = 225 m/s
= 225 m/s
Speed range (dc) will be calculated as follows.
dc = (250 - 200)
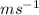
= 50
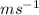
Boltzmann constant =
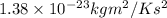
T = 300 K, mass of
 molecule =
molecule =
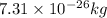
Calculate the fraction of molecules in range dc as follows.
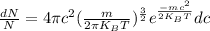
=
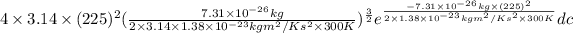
= 0.001917
Thus, we can conclude that fraction of
 molecules present are 0.001917.
molecules present are 0.001917.