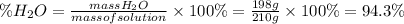Answer:
The mass percentage of the solvent is 94.3% by mass.
Step-by-step explanation:
We prepare a solution dissolving 11.9 g KMnO₄(solute) in 198g H₂O(solvent). The mass of the solution is:
mass of solution = mass of solute + mass of solvent
mass of solution = 11.9g + 198g = 210g
The mass percentage of the solvent is: