Answer:
a) Number-average molar mass: 163,700g/mol
Weight-average molar mass: 200,000g/mol
b) Number-average molar mass: 200,000g/mol
Weight-average molar mass: 233,300g/mol
Step-by-step explanation:
The number-average molar mass is based on mole% and weight-average molar mass is based on weight%, thus:
a) In equal proportions by weight:
number-average molar mass:
1g A×
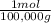 = 1x10⁻⁵ moles A
= 1x10⁻⁵ moles A
1g B×
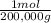 = 5x10⁻⁶ moles B
= 5x10⁻⁶ moles B
1g C×
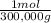 = 3,33x10⁻⁶ moles C
= 3,33x10⁻⁶ moles C
Total moles are:
1x10⁻⁵ A + 5x10⁻⁶ B + 3,33x10⁻⁶ C = 1,833x10⁻⁵ moles.
Percent A:
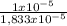 ×100 = 54,5%
×100 = 54,5%
Percent B:
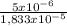 ×100 = 27,3%
×100 = 27,3%
Percent C:
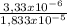 ×100 = 18,2%
×100 = 18,2%
number-average molar mass:
0,545*100,000g/mol + 0,273*200,000g/mol + 0,182*300,000g/mol = 163,700g/mol
weight-average molar mass:
0,333*100,000g/mol + 0,333*200,000g/mol + 0,333*300,000g/mol = 200,000g/mol
b) In molar ratio 1:1:1
weight-average molar mass:
1mol A×
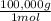 = 100,000g A
= 100,000g A
1mol B×
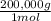 = 200,000g B
= 200,000g B
1mol C×
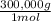 = 300,000g C
= 300,000g C
Total mass is:
100,000 A + 200,000 B + 300,000 C = 600,000 g.
Percent A:
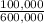 ×100 = 16,7%
×100 = 16,7%
Percent B:
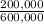 ×100 = 33,3%
×100 = 33,3%
Percent C:
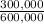 ×100 = 50,0%
×100 = 50,0%
Weight-average molar mass:
0,167*100,000g/mol + 0,333*200,000g/mol + 0,500*300,000g/mol = 233,300g/mol
number-average molar mass:
0,333*100,000g/mol + 0,333*200,000g/mol + 0,333*300,000g/mol = 200,000g/mol
I hope it helps!