Answer:
pH = 2,4
Step-by-step explanation:
pH is defined as a measure of acidity or alkalinity of a solution based on H⁺ concentration
The HCl dissociates in water thus:
HCl → H⁺ + Cl⁻
That means that moles of HCl are the same than H⁺.
0,35gHCl
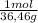 = 9,6x10⁻³ moles HCl ≡ moles H⁺
= 9,6x10⁻³ moles HCl ≡ moles H⁺
H⁺ molarity is:
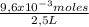 = 3,84x10⁻³M
= 3,84x10⁻³M
As pH = -log [H⁺]
pH = 2,4
I hope it helps!