Answer:
a) 6,69x10²² atoms H/cm³
b) 6,87x10²² atoms H/n·cm³
c) 1,50x10²¹ atoms H/cm³
Step-by-step explanation:
You can obtain the number of hydrogen atoms per cubic centimeter using density, molar mass and Avogadro's number, thus:
a)
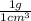 ×
×
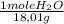 ×
×
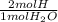 ×
×
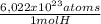 = 6,69x10²² atoms H/cm³
= 6,69x10²² atoms H/cm³
b) As oil formula is
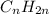
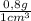 ×
×
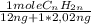 ×
×
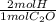 ×
×
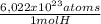 = 6,87x10²² atoms H/n·cm³ -It depends of n number-
= 6,87x10²² atoms H/n·cm³ -It depends of n number-
c)
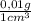 ×
×
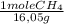 ×
×
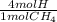 ×
×
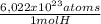 = 1,50x10²¹ atoms H/cm³
= 1,50x10²¹ atoms H/cm³
I hope it helps!